Abstract
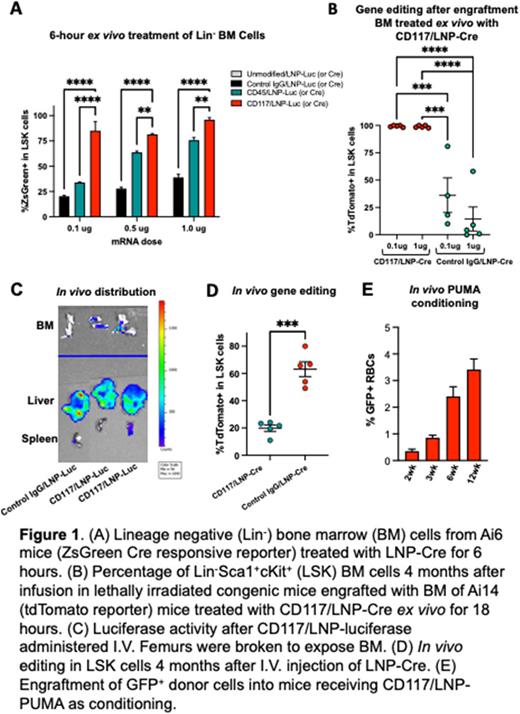
Gene therapy for non-malignant hematopoietic disorders (NMHD), such as hemoglobinopathies and immunodeficiencies, is currently done with ex vivo lentiviral transduction of hematopoietic stem cells (HSC) or electroporation of genome editing reagents. However, this approach still requires HSC-transplant (HSCT) with gene-modified, autologous HSC. Conditioning regimens, based upon high-dose chemotherapy or radiation, are required for depletion of existing HSC. Conditioning and HSCT carry significant acute and chronic systemic toxicities, including sterility and secondary malignancies due to accumulated DNA damage. We developed a novel lipid nanoparticle (LNP) encapsulating nucleoside-modified messenger RNA (mRNA) that is targeted to the stem cell factor receptor (CD117) by covalent attachment of an antibody. The efficacy of LNP targeted to HSC was evaluated utilizing a reporter system, whereby transfection of Cre recombinase mRNA effected genomic editing and fluorescent reporter activation. We utilized the Ai6, Ai9 and Ai14 mouse models, which have a loxP-flanked STOP cassette preventing transcription of a CAG promoter-driven fluorescent reporter gene at the ROSA26 locus. In ex vivo experiments, anti-CD117-LNP encapsulating Cre mRNA (CD117/LNP-Cre) produced 95% genome editing in LSK cells after 6 hours treatment (Fig 1A). This was superior to targeting CD45 (pan-hematopoietic marker) or Control IgG. To evaluate multipotency in cells edited by CD117/LNP-Cre, we transplanted lethally irradiated, congenic C57BL/6 CD45.1 recipient mice with Ai14 BM cells treated ex vivo with increasing doses (up to 1mg) of CD117/LNP-Cre and Control IgG/LNP-Cre. The % of CD117/LNP-Cre-mediated tdTomato+ Ai14 cells in recipient mice increased with time post-HSCT, reaching ~99% of donor cells, consistent with genome editing of multipotent HSC (Fig 1B). These HSC were found to be self-renewing utilizing secondary transplantation.
Given the near complete targeting of LT-HSC ex vivo, we administered CD117/LNP encapsulating luciferase mRNA and observed luciferase activity in the bone marrow (Fig 1C). The absolute rate of HSC targeting after a single in vivo CD117/LNP-Cre (5mg) treatment was 64% of bone marrow cells four months post treatment (Fig 1D). This was 4.2-fold higher than seen with Control IgG/LNP-Cre at the same dose. To prove that these modifications targeted functional HSC, transplants were generated by injecting irradiated congenic (C57BL/6 CD45.1) recipients with BM from two mice treated in vivo with 5 µg dose of CD117 or control IgG/LNP-Cre. Donor BMs isolated 4-month after HSCT maintained the same level of editing as donor mice, demonstrating that CD117/LNP-Cre efficiently targets and induces Cre mediated genome editing in true HSC in vivo. Although a proportion of cells in the liver and lung were targeted by CD117/LNP-Cre, offspring from in vivo treated male mice (from both control IgG/ and CD117/LNP-mRNA treatment groups) did not express tdTomato, indicating absence of germ cell targeting in vivo.
The ability to target HSC in vivo offers a route to non-genotoxic conditioning regiment for HSCT, especially for diseases associated with DNA damage repair defects. Therefore, we sought to test the ability of CD117/LNP to deplete BM cells using pro-apoptotic mRNA given human and mouse HSC dependency on the anti-apoptotic gene Mcl1. We tested a variety of pro-apoptotic mRNAs that act within this pathway. Among those genes, treatment with CD117/LNP encapsulating PUMA mRNA (CD117/LNP-PUMA) reduced BM and LSK viability in vitro. We tested CD117/LNP-PUMA induced depletion in vivo, followed by transplantation of GFP+ BM cells. Given non-specific LNP uptake in the liver, we incorporated a liver specific miRNA binding site (mir-122) into the 3'UTR of the PUMA mRNA to decrease expression in hepatocytes. C57BL/6 recipients received CD117/LNP-PUMA at 0.05 mg/kg mRNA dose and 106 GFP+ C57BL/6 BM cells on day 7. The level of engraftment (up to 12 weeks) confirmed progressive increase of GFP+ donor cells (Fig 1E).
In conclusion, these findings may revolutionize medicine in two ways. First, the cure of NMHD disorders will be attainable with a simple infusion that corrects the causative molecular defect. Second, effecting cell-type specific state changes in vivo with minimal risk will allow previously impossible manipulations of physiology.
LB, TEP, MT: first Authors. SR, HP: corresponding Authors.
Disclosures
Weissman:AexeRNA: Other: Co-founder; BioNTech: Research Funding. Rivella:BVF Partners L.P: Consultancy; Cambridge Healthcare Res: Consultancy; Catenion: Consultancy; Celgene: Consultancy; Disc Medicine: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; First Manhattan Co: Consultancy; FORMA: Consultancy; Ghost Tree Capita: Consultancy; Incyte: Membership on an entity's Board of Directors or advisory committees; Ionis Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Keros Therapeutics: Consultancy; MeiraGTx: Consultancy; Noble insight: Consultancy; Protagonist Therapeutics: Consultancy; Rallybio, LLC: Consultancy; Sanofi Aventis U.S: Consultancy; Slingshot Insight: Consultancy; Techspert.io: Consultancy; venBio Select LLC: Consultancy; Vifor: Membership on an entity's Board of Directors or advisory committees. Parhiz:University of Pennsylvania: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal